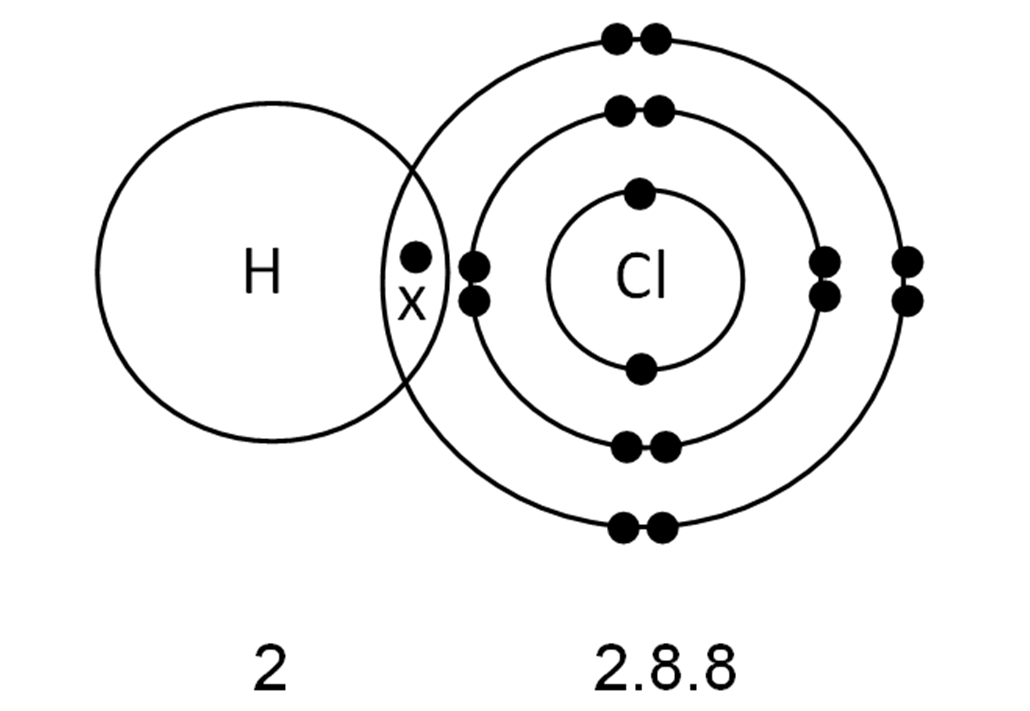
Chlorine Expanded Valence Shell . learn how to assign lewis dot symbols to elements with less than an octet of electrons, such as bcl3. It is possible to move one electron from chlorine. Learn the electronic explanation, the duplet rule,. expanded valence shells occur most often when the central atom is bonded to small electronegative atoms, such as f, cl and o. See examples of lewis structures for no, sf6, bcl3,. $\ce{o}$ atom has 6 electrons at the valence shell with four orbitals. See examples of molecules with odd, expanded, and deficient valence shells. learn why and how some molecules or ions have an odd number of electrons, more than an octet, or less than an octet. learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. learn about the three types of exceptions to the octet rule in covalent bonding:
from chemistry98.blogspot.com
It is possible to move one electron from chlorine. Learn the electronic explanation, the duplet rule,. learn about the three types of exceptions to the octet rule in covalent bonding: See examples of lewis structures for no, sf6, bcl3,. See examples of molecules with odd, expanded, and deficient valence shells. learn why and how some molecules or ions have an odd number of electrons, more than an octet, or less than an octet. learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. expanded valence shells occur most often when the central atom is bonded to small electronegative atoms, such as f, cl and o. learn how to assign lewis dot symbols to elements with less than an octet of electrons, such as bcl3. $\ce{o}$ atom has 6 electrons at the valence shell with four orbitals.
Chem Easy Formation of covalent bond in chlorine molecule
Chlorine Expanded Valence Shell $\ce{o}$ atom has 6 electrons at the valence shell with four orbitals. learn how to assign lewis dot symbols to elements with less than an octet of electrons, such as bcl3. expanded valence shells occur most often when the central atom is bonded to small electronegative atoms, such as f, cl and o. learn about the three types of exceptions to the octet rule in covalent bonding: learn why and how some molecules or ions have an odd number of electrons, more than an octet, or less than an octet. Learn the electronic explanation, the duplet rule,. See examples of molecules with odd, expanded, and deficient valence shells. It is possible to move one electron from chlorine. $\ce{o}$ atom has 6 electrons at the valence shell with four orbitals. learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. See examples of lewis structures for no, sf6, bcl3,.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Expanded Valence Shell $\ce{o}$ atom has 6 electrons at the valence shell with four orbitals. expanded valence shells occur most often when the central atom is bonded to small electronegative atoms, such as f, cl and o. Learn the electronic explanation, the duplet rule,. learn about the three types of exceptions to the octet rule in covalent bonding: learn. Chlorine Expanded Valence Shell.
From byjus.com
Find out the number of valence electrons present in chlorine. Chlorine Expanded Valence Shell It is possible to move one electron from chlorine. See examples of molecules with odd, expanded, and deficient valence shells. learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. See examples of lewis structures for no, sf6, bcl3,. learn about the three types of exceptions. Chlorine Expanded Valence Shell.
From www.sliderbase.com
Molecules with Expanded Valence Shells Chlorine Expanded Valence Shell See examples of molecules with odd, expanded, and deficient valence shells. Learn the electronic explanation, the duplet rule,. $\ce{o}$ atom has 6 electrons at the valence shell with four orbitals. learn about the three types of exceptions to the octet rule in covalent bonding: expanded valence shells occur most often when the central atom is bonded to. Chlorine Expanded Valence Shell.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Shutterstock Chlorine Expanded Valence Shell See examples of molecules with odd, expanded, and deficient valence shells. expanded valence shells occur most often when the central atom is bonded to small electronegative atoms, such as f, cl and o. It is possible to move one electron from chlorine. learn about the three types of exceptions to the octet rule in covalent bonding: learn. Chlorine Expanded Valence Shell.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Expanded Valence Shell See examples of lewis structures for no, sf6, bcl3,. It is possible to move one electron from chlorine. expanded valence shells occur most often when the central atom is bonded to small electronegative atoms, such as f, cl and o. learn how to assign lewis dot symbols to elements with less than an octet of electrons, such as. Chlorine Expanded Valence Shell.
From www.doubtnut.com
In electron dot structure, The valence shell electrons are represented Chlorine Expanded Valence Shell learn about the three types of exceptions to the octet rule in covalent bonding: $\ce{o}$ atom has 6 electrons at the valence shell with four orbitals. expanded valence shells occur most often when the central atom is bonded to small electronegative atoms, such as f, cl and o. Learn the electronic explanation, the duplet rule,. See examples. Chlorine Expanded Valence Shell.
From dyzogfqveco.blob.core.windows.net
Chlorine Valence Electrons Charge at William Predmore blog Chlorine Expanded Valence Shell See examples of molecules with odd, expanded, and deficient valence shells. It is possible to move one electron from chlorine. learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. $\ce{o}$ atom has 6 electrons at the valence shell with four orbitals. expanded valence shells. Chlorine Expanded Valence Shell.
From byjus.com
In the electron dot structure, the valence shell electrons are represented by crosses or dots. a Chlorine Expanded Valence Shell learn about the three types of exceptions to the octet rule in covalent bonding: learn why and how some molecules or ions have an odd number of electrons, more than an octet, or less than an octet. Learn the electronic explanation, the duplet rule,. learn how to assign lewis dot symbols to elements with less than an. Chlorine Expanded Valence Shell.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Expanded Valence Shell learn why and how some molecules or ions have an odd number of electrons, more than an octet, or less than an octet. Learn the electronic explanation, the duplet rule,. expanded valence shells occur most often when the central atom is bonded to small electronegative atoms, such as f, cl and o. $\ce{o}$ atom has 6 electrons. Chlorine Expanded Valence Shell.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence electrons in chlorine Chlorine Expanded Valence Shell learn how to assign lewis dot symbols to elements with less than an octet of electrons, such as bcl3. learn about the three types of exceptions to the octet rule in covalent bonding: Learn the electronic explanation, the duplet rule,. See examples of lewis structures for no, sf6, bcl3,. expanded valence shells occur most often when the. Chlorine Expanded Valence Shell.
From www.slideserve.com
PPT Chemistry XL14A Chemical bonds PowerPoint Presentation, free download ID2313701 Chlorine Expanded Valence Shell learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. $\ce{o}$ atom has 6 electrons at the valence shell with four orbitals. See examples of lewis structures for no, sf6, bcl3,. learn about the three types of exceptions to the octet rule in covalent bonding:. Chlorine Expanded Valence Shell.
From www.sliderbase.com
Molecules with Expanded Valence Shells Chlorine Expanded Valence Shell See examples of molecules with odd, expanded, and deficient valence shells. learn how to assign lewis dot symbols to elements with less than an octet of electrons, such as bcl3. Learn the electronic explanation, the duplet rule,. See examples of lewis structures for no, sf6, bcl3,. learn why and how some molecules or ions have an odd number. Chlorine Expanded Valence Shell.
From studylib.net
Expanded valence shells (extended octets) Chlorine Expanded Valence Shell See examples of molecules with odd, expanded, and deficient valence shells. $\ce{o}$ atom has 6 electrons at the valence shell with four orbitals. It is possible to move one electron from chlorine. learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. learn about the. Chlorine Expanded Valence Shell.
From brokeasshome.com
Periodic Table Chlorine Electrons Chlorine Expanded Valence Shell Learn the electronic explanation, the duplet rule,. learn how to assign lewis dot symbols to elements with less than an octet of electrons, such as bcl3. learn why and how some molecules or ions have an odd number of electrons, more than an octet, or less than an octet. expanded valence shells occur most often when the. Chlorine Expanded Valence Shell.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Expanded Valence Shell Learn the electronic explanation, the duplet rule,. learn why and how some molecules or ions have an odd number of electrons, more than an octet, or less than an octet. learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. learn about the three types. Chlorine Expanded Valence Shell.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Expanded Valence Shell learn how to assign lewis dot symbols to elements with less than an octet of electrons, such as bcl3. See examples of molecules with odd, expanded, and deficient valence shells. learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. learn why and how some. Chlorine Expanded Valence Shell.
From cebhjyoy.blob.core.windows.net
Chlorine Has 7 Valence Electrons. Why Would It Love To Gain Another Electron at Vicky Mitchell blog Chlorine Expanded Valence Shell learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. learn about the three types of exceptions to the octet rule in covalent bonding: See examples of lewis structures for no, sf6, bcl3,. learn why and how some molecules or ions have an odd number. Chlorine Expanded Valence Shell.
From www.chegg.com
Solved Chlorine is a period 3 element and can therefore have Chlorine Expanded Valence Shell Learn the electronic explanation, the duplet rule,. learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. See examples of molecules with odd, expanded, and deficient valence shells. expanded valence shells occur most often when the central atom is bonded to small electronegative atoms, such as. Chlorine Expanded Valence Shell.